Arsenic has a complex and dangerous history. It has many forms, and it can even transform from one to another just as a chameleon changes its colours.
At the atomic level, arsenic easily exchanges ions, making it capable of existing in many different chemical forms. It can combine readily with many other elements. Its shape shifting nature makes it a tough contaminant to remove from our water supply.
Arsenic combined with carbon forms organic arsenic, but can as easily exist in an inorganic form.
Dominant inorganic forms are arsenite, with a valency of 3, and arsenate, with a valency of 5. (Valency measures the ability of a compound to combine with other elements). Inorganic forms of arsenic are generally considered to be the most toxic.
Because it takes so many different forms it is even difficult to visually differentiate. It’s a rather weird chemical that even sometimes may act as a metal. It can take the form of a crystalline powder or brittle, silver and metal-like metaloid. Arsenic can be found naturally occurring in rocks and soil around the globe. (It’s in our local water supply but authorities tell us it’s ‘not enough to matter’!) Concentrations become higher in some areas through weathering and human-related activities such as mining, fossil fuel combustion and pesticide use.
So.. is it hazardous?
Oh yes. Hazardous falls short as a description of arsenic’s many disguises. It is one of history’s most notorious poisons. It was used so often as a homicidal weapon within competing medieval royal families that any royal death was suspected to be arsenic poisoning.
- Joseph Graziano’s researched it at Columbia University (2004) He showed that chronic arsenic exposure contributes to reduced cognitive function in children.
- Long-term exposure just to small amounts of arsenic in drinking water can cause cancer of the skin, lungs, bladder and kidneys.
- It interferes with circulation and normal red blood cell reproduction.
- In Taiwan, arsenic has been linked to blackfoot disease, where ingesting arsenic through drinking water over time causes decreased circulation in the feet and lower legs, eventually leading to gangrene.
- In Bangladesh, large scale tube well installation in the 1970s and 1980s to provide freshwater for rural communities inadvertently tapped into arsenic-rich groundwater, leading to the mass poisoning of millions of people.
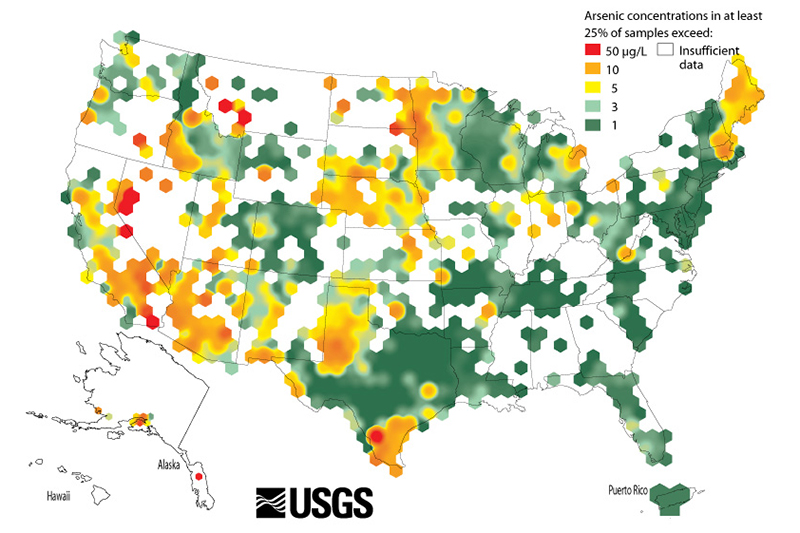
It is estimated that as many as 25 million Americans, many of them private water well owners, drink water from a source above the current EPA limit for arsenic.
What can you do to remove arsenic from your drinking water?
Arsenic is so complex, so removal is a difficult process.
Three water treatment systems noted for arsenic removal: reverse osmosis (RO), adsorption media and distillation. RO systems and adsorption media are only partially effective at arsenic removal while distillation is clearly superior at arsenic removal from water.
Several forms of arsenic can be removed to some degree by RO filtration systems, but RO does not remove Arsenic 3 unless it has been pretreated with an oxidizing agent such as chlorine or ozone. In addition to the cost and trouble of pretreatment, RO systems lose effectiveness over time, and with temperature change they require frequent filter membrane replacements and waste copious amounts of rejected water. We’ve struggled to recommend RO systems to people wanting the purest of pure water but the physical maintenance required means most RO’s cannot live up to what is expected of them
Adsorption media water treatment systems offer promise for large-scale arsenic removal. Adsorption media use metal oxides distributed over a flow-bed to form a chemical bond with arsenic in the water. The metal-oxide media are later discarded. In California, the spent media must be disposed of as hazardous waste. Pretreatment is required to remove suspended solids, iron and manganese from the water. Regular media replacement is required because these systems lose efficiency over time. In the UltraStream we use KDF85, a patented broad spectrum media that removed or neutralises heavy metals.
In the case of arsenic and most other contaminants, distillation results in a 99.9 percent contaminant reduction. In Arizona, where arsenic in groundwater is a major concern, the University of Arizona has called distillation “the most reliable water treatment process for arsenic removal” (The University of Arizona Cooperative Extension publication). It certainly is, and it is simple, but not popular because it relies on the electricity to evaporate all the water to distill it, and the water cannot be immediately consumed until it cools. Opponents of distillation will commonly point to its inability to remove volatile organic gases such as chlorine with a lower boiling point than water.
